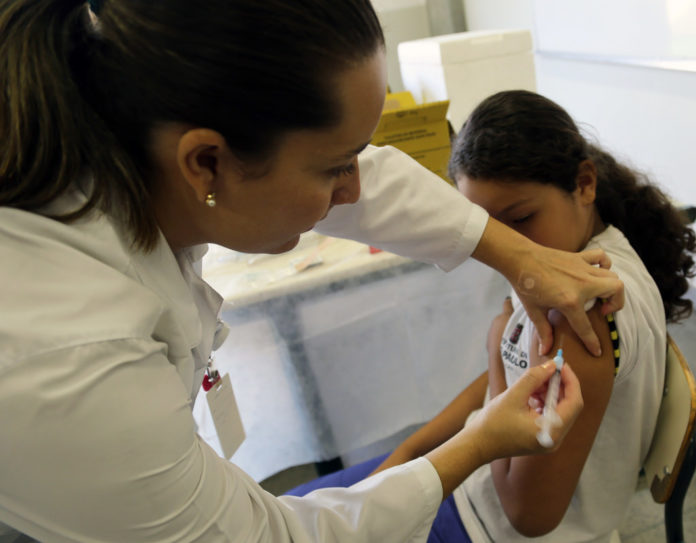
The US Food and Drug Administration expanded the coverage of HPV vaccine to include men and women aged 27 to 45 years
The US Food and Drug Administration has expanded the recommended coverage of HPV vaccine, to include men and women aged 27 to 45 years. The vaccine can prevent some forms of cervical cancers.
Human papillomavirus is the most common sexually transmitted infection worldwide, affecting 50%-75% of sexually active people.
HPV vaccine is yet to be included in the universal immunisation programme in India due to some discordant voices questioning the safety and efficacy of the vaccine. This expanded approval from one of the most prestigious drug regulatory agencies would add yet another dimension to that debate.
The vaccine is Gardasil 9, the recombinant 9-valent vaccine, made by Merck & Co. It had been previously approved for minors and people up to age 26. It protects against 9 strains of Human Papillomavirus (HPV), those most likely to cause cancers and genital warts.
Gardasil 9, provides protection against HPV, which can also cause cancers of the vulva, anus, penis and parts of the throat. Most adults encounter at least one strain at some point in their lives.
Gardasil was 88 percent effective in the prevention of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer
“Today’s approval represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” said Dr. Peter Marks, director of the F.D.A.’s Center for Biologics Evaluation and Research, in a statement.
The FDA’s approval of Gardasil 9 in women 27 through 45 years of age is based on the results of a study in 3,200 women 27 through 45 years of age, followed for an average of 3.5 years. Gardasil was 88 percent effective in the prevention of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine. New data on long term follow-up from this study were also considered.
Effectiveness of Gardasil 9 in men 27 through 45 years of age is inferred from the data in women, as well as efficacy data from Gardasil in younger men (16 through 26 years of age) and immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months.
The safety of Gardasil 9 was evaluated in about a total of 13,000 males and females. The most commonly reported side effects of the vaccine include pain at the injection site, swelling, redness and headaches.
If a person has already been exposed to a particular strain of HPV, the vaccine will not work against that strain. Vaccination has therefore, been strongly recommended for young people before they become sexually active. But even if someone has been already exposed to a few strains, he/she can still gain protection against the strains that they have not encountered.









[…] (adsbygoogle = window.adsbygoogle || []).push({}); Source link […]
Comments are closed.