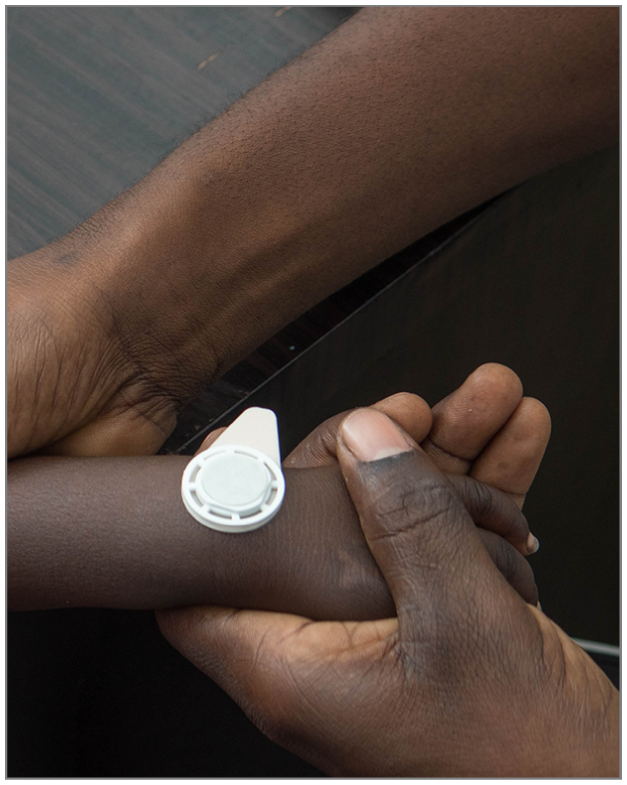
In a trial conducted in Gambia it was found that microneedle patches compare favourably with subcutaneous injections in immunogenicity
In first data coming in on the safety and immunogenicity of microneedle patches (MNP) for the delivery of measles rubella vaccination, a trial in Gambia has shown that they are favourably comparable to subcutaneous injections.
MNPs are crucial to achieve universal vaccine coverage in low resource or difficult settings. Vaccine Innovation Prioritization Strategy, a consortium including WHO, UNICEF, and Gavi, the Vaccine Alliance, recently ranked the development of MNP as the highest global priority for achieving equity of vaccine coverage in low-income and middle-income countries. The patch needs to be kept on for five minutes.
The researchers wrote in The Lancet: “MRV administration by MNP is well tolerated and safe in adults, toddlers, and infants. The immunogenicity of the vaccine delivered by MNP is similar to the immunogenicity of the vaccine when administered subcutaneously by needle and syringe. MNPs are considered to be of highest priority for overcoming barriers to immunisation in LMICs and to achieving measles and rubella elimination. This phase 1/2 trial data supports their accelerated development for this purpose as well as ongoing work to apply the technology for the delivery of other priority vaccines.” MRV stands for measles rubella vaccine. The study was funded by the Bill and Melinda Gates Foundation.
Despite significant increase in the coverage of the measles vaccine, India continues to also have a very high disease burden, thanks to the large birth cohort – about 25 million annually. According to the WHO, India vaccinated over 348 million children between 2017 and March 2023. Measles cases dropped by 62% between 2017 and 2021, from 10.4 to 4 cases per million population, while rubella cases decreased by 48%, from 2.3 to 1.2 cases per million population