US scientists have zeroed in on a promising candidate for a Zika vaccine after an investigational Zika purified inactivated virus (ZPIV) was found to be safe and also generated an immune response in participants during three Phase 1 clinical trials. Scientists at the Walter Reed Army Institute of Research (WRAIR), part of the U.S. Department of Defense, are developing the vaccine and leading one of the trials, according to communication from the National Institutes of Health.
“A vaccine is urgently needed to help prevent Zika infection, which can cause birth defects and other developmental abnormalities in babies born to infected women, as well as a constellation of other health problems in infected adults and children. We are encouraged by initial clinical trial results that indicate the ZPIV vaccine is safe and immunogenic, data that support additional clinical testing of the vaccine to determine its ability to prevent Zika virus infection.,” says National Institute of Allergy and Infectious Diseases director Anthony S. Fauci.
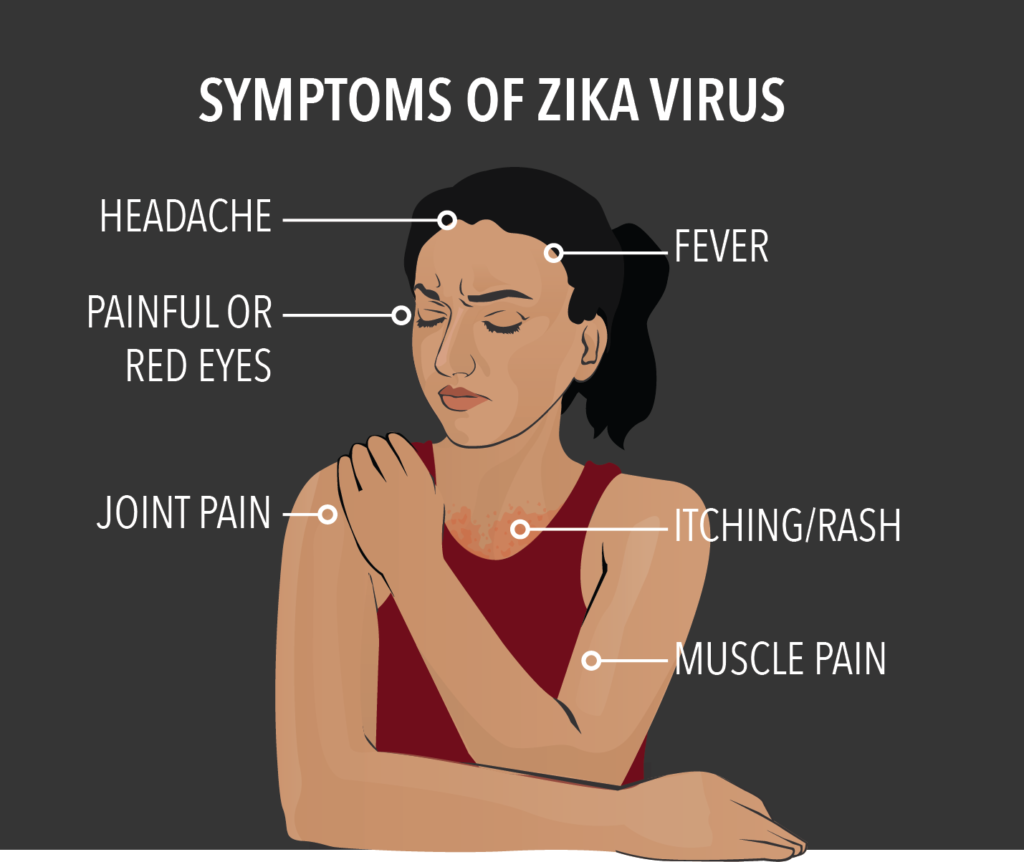
Zika is spread mostly by the bite of an infected Aedes species mosquito (Ae. aegypti and Ae. albopictus). These mosquitoes bite during the day and night. It can be passed from a pregnant woman to her child and can cause birth defects, the most well known of which is microencephaly or babies born with small heads and resultant brain deficiencies. There is currently no medicine or vaccine for Zika. Earlier this year, the NDA government in India was accused of deliberate suppression of facts when a World Health Organisation press statement in May revealed how for months the fact that three cases of Zika virus infection had been detected in Gujarat, was kept under wraps. One case dated back to November 9, 2016, one was detected during routine surveillance between January 6-12 2017 and one between February 10-16, 2017. The suppression was unusual because Zika requires a number of public health measures and awareness is key to their implementation.
Meanwhile, the ZPIV vaccine candidate contains whole Zika virus particles that have been inactivated and therefore cannot replicate and cause disease in humans. However, because the protein shell of the inactivated virus remains intact, it can be recognized by the immune system. Of the 67 adult participants in the initial studies, 55 received the investigational vaccine and 12 received a placebo. The investigational vaccine was administered with an adjuvant (a compound that helps induce a stronger immune response) containing aluminum salts. All participants received two intramuscular injections of the same dose four weeks apart. The trial was double-blinded, meaning neither the investigators nor the participants knew who received a placebo.
Investigators tested participants’ blood samples periodically and detected antibodies to Zika virus in more than 90 percent of individuals who received the experimental vaccine, within four weeks after the last dose.











