India is all set to roll out a National Programme for Control of Viral Hepatitis. The Expenditure Finance Committee has approved a budget of Rs 600 crore for the next three years. It is expected to start in the 2018-19 financial year.
Special focus of the programme will be on Hepatitis C for which an anti-viral that costs between $63000-94000 for the full course in USA and Europe would be available free in all government health setups in the country. At an estimated burden of 12 million (1.2 crore), India’s Hepatitis C load is about six times the HIV/AIDS burden and currently the Union government does not spend anything on screening and control of the disease.
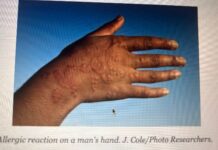
Like HIV, Hepatitis C too is a blood borne virus affecting the liver that spreads injection drug use, unsafe injection practices, unsafe health care, and the transfusion of unscreened blood and blood products but is not sexually transmitted unlike Hepatitis B for which India has already included a vaccine in its universal immunisation basket. Sexual risk of Hepatitis C transmission is low (0-3%) when having sex with a Hepatitis c infected partner. Very negligible amount of the virus is present in semen or vaginal fluid
There is no vaccine for Hepatitis C. A significant number of people infected with the Hepatitis C virus go on to develop cirrhosis or liver cancer – diseases that cause bulk of the 399000 deaths that annually occur the world over from Hepatitis C, according to WHO estimates.
For starters the ministry is looking at screening of vulnerable groups such as injectable drug users and people who underwent surgeries/blood transfusion before 2002 – when the protocols for screening were not as robust. Taking off from similar programmes in Punjab and Haryana where state governments have negotiated Sofosbuvir and Daclatasvir/Ledipasvir/Velpatasvir prices in the Rs 7000-8000 range, the Union government is already in informal talks with various Indian companies manufacturing Sofosbuvir on behalf of the American pharma giant Gilead on the price points for the drug for a national programme.
Sofosbuvir is recommended under the new WHO treatment guidelines for Hepatitis C. Resolution WHA67.6 adopted by the 67th World Health Assembly in 2014, requested the Director-General “to work with national authorities upon their request, to promote comprehensive, equitable access to prevention, diagnosis and treatment for viral hepatitis” and “to assist Member States to ensure equitable access to quality, effective, affordable and safe hepatitis B and HCV treatments and diagnostics, in particular in developing countries”.