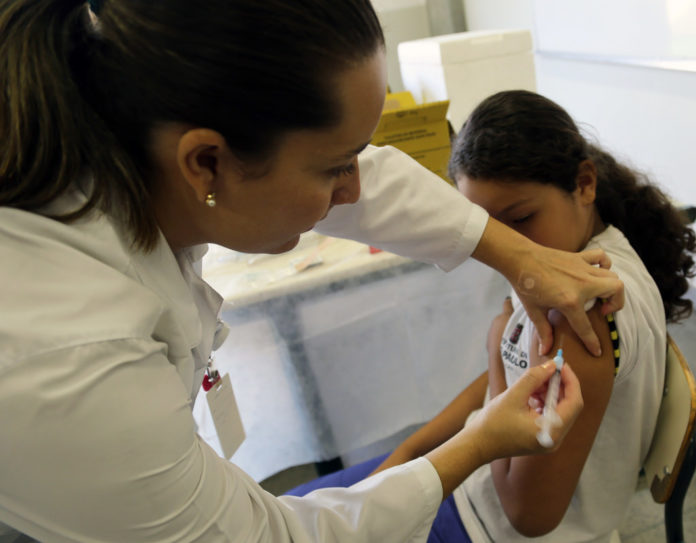
Cervical cancer is the second most common type of cancer in females in India
The highest decision making body on vaccines – the National Technical Advisory Group of Immunisation (NTAGI) – is set to meet on December 19 and sources in the Union health ministry say that a recommendation made by a sub-group for the introduction of the Human Papilloma Virus (HPV) is likely to be taken up by the committee in its next meeting. HPV was on the agenda for the NTAGI meeting that was to happen on December 6 but the meeting got postponed. However the matter is already controversial with Swadeshi Jagran Manch, the economic wing of the Rashtriya Swayamsewak Sangh, having written to the prime minister opposing the move.
According to information given out by the US National Institutes of Health (NIH), Human papillomaviruses (HPVs) are a group of more than 200 related viruses. Many of them can easily spread through direct sexual contact, from the skin and mucous membranes of infected people to the skin and mucous membranes of their partners. They can be spread by vaginal, anal, and oral sex. Other HPV types are responsible for non-genital warts, which are not sexually transmitted.
Cervical cancer is the second most common type of cancer in females in India. According to the National Health Profile 2017, there were 97909 cases of cervical cancer in 2015. In 2013 the figure was 92,731. It is projected to go up to 104060 by 2020. HPV is the commonest cause but not the sole cause of cervical cancer. Not all HPV infections go on to become cancerous. As some oncologists put it, it is a necessary but not a sufficient cause.
In 2015 the Indian health ministry had asked a sub-group of NTAGI to look at the pros and cons of introducing HPV into the routine immunisation programme. That sub group recommended its inclusion. In its next meeting on December 19, NTAGI is expected to deliberate on that recommendation.
While unethical clinical trials in Andhra Pradesh and Gujarat some time back brought a bad name for HPV, the real concern in its inclusion in the government immunisation schedule is the cost. At Rs 2000-3000 per dose and the requirement of three doses, the cost per adolescent girl could be in the range of Rs 10,000. Though the global vaccine alliance GAVI has pledged some support to India for HPV, is will be of a temporary nature and the long term expenses will be a big consideration before a decision on the vaccine is taken, say senior health ministry officials.